

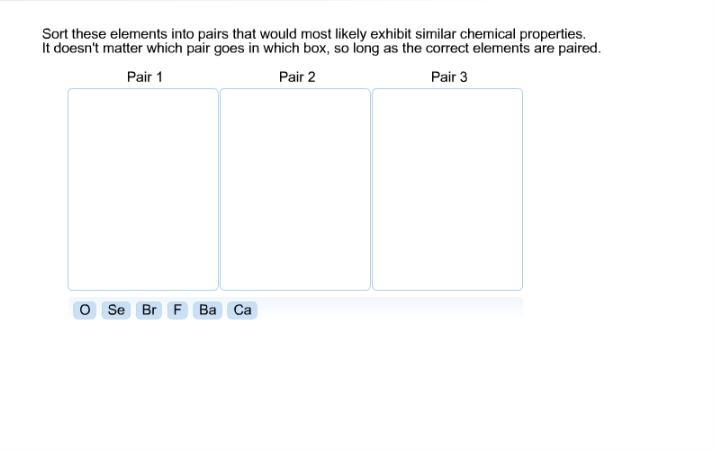
So as we know that then to be loathing down the group. And we have to arrange them in increasing order of atomic radius. radius of F is expressed in terms of covalent radius and atomic radius of Ne. That is oxygen, sulfur, selenium, thallium. Please report any accidental mistake in the above statistics on chemical elements. Arrange the elements N, P, O and S in the order of : (i) increasing first. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals.Ĭlick here: for a schematic overview of the periodic table of elements in chart form a group the elements become more reactive this is because the atomic radius increases the. Please note that the elements do not show their natural relation towards each other as in the Periodic system. (b) Elements of group 17, in decreasing order of reactivity. The first chemical element is Cesium and the last one is Helium.
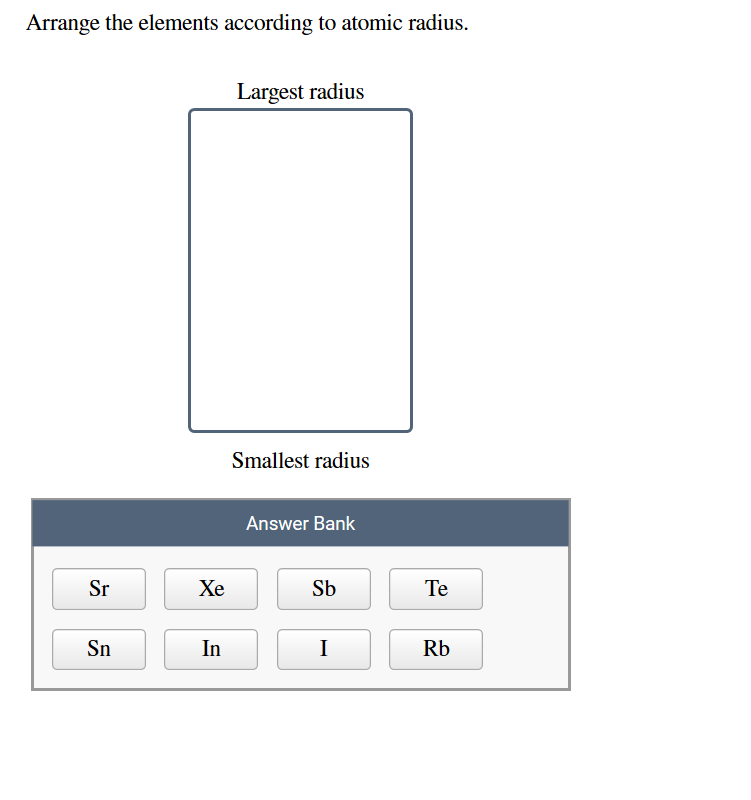
The chemical elements ofįor chemistry students and teachers: The tabular chart on the right is arranged by Ionization energy. These two trends allow us to conclude that the greatest atomic radius is found in the element in the bottom left corner of the periodic table. This list contains the 118 elements of chemistry. Phone: +31 152 610 900 of the innovation award 2017Ĭhemical elements listed by ionization energy The elements of the periodic table sorted by ionization energyĬlick on any element's name for further information on chemical properties, environmental data or health effects.Plant Inspection & Process Optimalisation.


 0 kommentar(er)
0 kommentar(er)
