
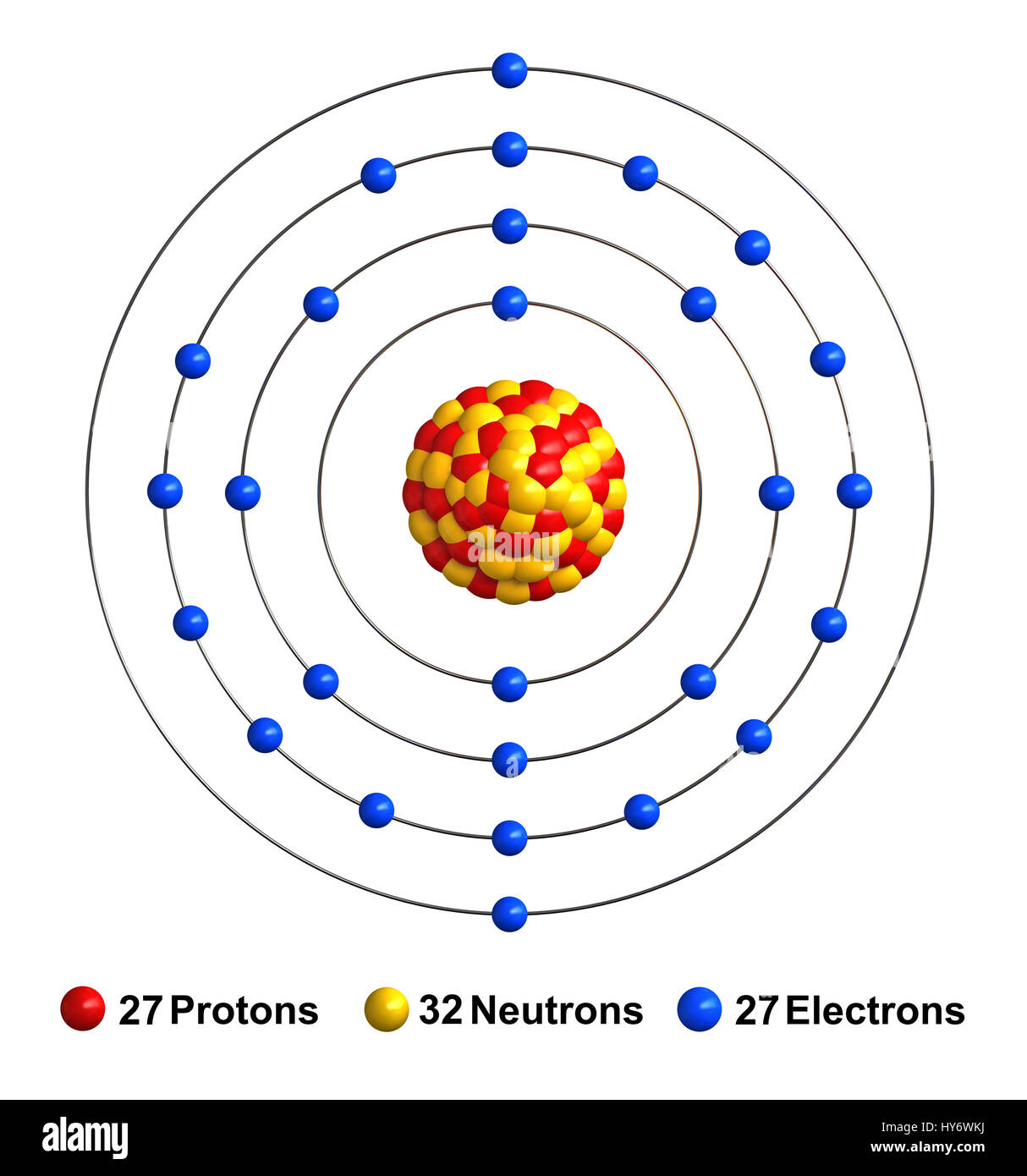
- COBALT ELECTRON CONFIGURATION HOW TO
- COBALT ELECTRON CONFIGURATION SERIES
- COBALT ELECTRON CONFIGURATION FREE
The values of ℓ = 0, 1, 2, and 3 correspond to the orbit s, p, d, and f, respectively. An atom with an nth electron shell can hold 2n^2 electrons, which is the first shell that can hold 2 electrons, the second shell can hold 8 electrons, and so on.Ī subshell is a set of states, which are defined by the total azimuth quantum number “l” in the shell. Shells and Subshells:Įlectron shells are a set of feasible states that have the same principal quantum number n (the number before the letter on the orbital) that the electron can occupy. However, an Online Angular Velocity Calculator allows you to determine the angular velocity of the body in motion on a circular path.
COBALT ELECTRON CONFIGURATION FREE
COBALT ELECTRON CONFIGURATION SERIES
For atoms, the standard notation consists of a series of atomic subshell labels (for example, phosphorus sequence of notation is 1s, 2s, 2p, 3s, 3p), where the number of electrons assigned to each subshell is used as a superscript.
COBALT ELECTRON CONFIGURATION HOW TO
Usually, Physicists and chemists use the isotope notation calculator to refer how to calculate electronic configurations of molecules and atoms. For example, electron configuration of Phosphorus (P) is 1s^2 2s^2 2p^6 3s^2 3p^3. It also describes every electron as moving freely in an orbital, in an average field generated by other orbitals. In quantum chemistry and atomic physics, the electron configuration of an atom or molecule describes the distribution of electron distribution mnemonics in different atomic or molecular orbitals. Read on to understand abbreviated electron configuration, shells, subshell, and how to find electron configuration of an atom or element. This valence electron calculator displays the abbreviated configuration and the atomic number of each element. Hardness of Cobalt - Tests to Measure of Hardness of Element Mohs HardnessĬobalt is Conductor of electricity.An online condensed electron configuration calculator helps you to determine the electron configuration of any element. Refer to below table for Cobalt Physical Properties DensityĨ.9 g/cm3(when liquid at m.p density is $7.75 g/cm3) All possible symmetric arrangements of particles in three-dimensional space are described by the 230 space groups (219 distinct types, or 230 if chiral copies are considered distinct. The symmetry properties of the crystal are described by the concept of space groups. The positions of the atoms inside the unit cell are described by the set of atomic positions ( x i, y i, z i) measured from a reference lattice point. The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges Lattice Constants (a, b and c) aĪnd the angles between them Lattice Angles (alpha, beta and gamma). The unit Cells repeats itself in three dimensional space to form the structure. The Crystal structure can be described in terms of its unit Cell.

The solid state structure of Cobalt is Simple Hexagonal.


 0 kommentar(er)
0 kommentar(er)
